Objective
- How To Tell If A Carbon Is Sp3 Hybridized
- Sp3 Hybridized Carbon Atom
- Sp3 Hybridized Carbon Has
- Sp3 Hybridized Carbon Examples
After completing this section, you should be able to apply the concept of hybridization to atoms such as N, O, P and S explain the structures of simple species containing these atoms.
How To Tell If A Carbon Is Sp3 Hybridized
Key Terms
Make certain that you can define, and use in context, the key term below.
- lone pair electrons
Study Notes
In general, an atom with all single bonds is an sp3 hybridized. The best example is the alkanes. All the carbon atoms in an alkane are sp3 hybridized with tetrahedral geometry. The carbons in alkenes and other atoms with a double bond are often sp2 hybridized and have trigonal planar geometry. Carbon-Carbon bonds: Hybridization Peschel obtained, being a number between 0 and 1. With this describtion one can specify the ratio between sp2- and sp3-orbitals. All these characteristica are strongly dependent on the diameter and the chiral. Carbon - sp3 hybridization When carbon is bonded to four other atoms (with no lone electron pairs), the hybridization is sp 3 and the arrangement is tetrahedral. Notice the tetrahedral arrangement of atoms around carbon in the two and three-dimensional representations of methane and ethane shown below.
Nitrogen is frequently found in organic compounds. As with carbon atoms, nitrogen atoms can be sp3-, sp2– or sp‑hybridized.
Sp3 Hybridized Carbon Atom
Note that, in this course, the term “lone pair” is used to describe an unshared pair of electrons.
The valence-bond concept of orbital hybridization can be extrapolated to other atoms including nitrogen, oxygen, phosphorus, and sulfur. In other compounds, covalent bonds that are formed can be described using hybrid orbitals.
Methylamine
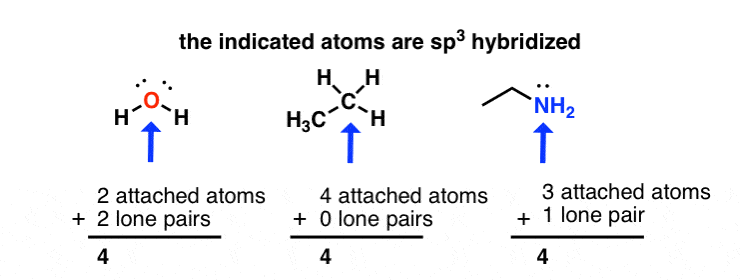
The nitrogen is sp3hybridized which means that it has four sp3 hybrid orbitals. Two of the sp3hybridized orbitals overlap with s orbitals from hydrogens to form the two N-H sigma bonds. One of the sp3 hybridized orbitals overlap with an sp3 hybridized orbital from carbon to form the C-N sigma bond. The lone pair electrons on the nitrogen are contained in the last sp3 hybridized orbital. Due to the sp3 hybridization the nitrogen has a tetrahedral geometry. However, the H-N-H and H-N-C bonds angles are less than the typical 109.5o due to compression by the lone pair electrons.
Methanol
The oxygen is sp3hybridized which means that it has four sp3 hybrid orbitals. One of the sp3hybridized orbitals overlap with s orbitals from a hydrogen to form the O-H signma bonds. One of the sp3 hybridized orbitals overlap with an sp3 hybridized orbital from carbon to form the C-O sigma bond. Both the sets of lone pair electrons on the oxygen are contained in the remaining sp3 hybridized orbital. Due to the sp3 hybridization the oxygen has a tetrahedral geometry. However, the H-O-C bond angles are less than the typical 109.5o due to compression by the lone pair electrons.
Methanol
Methyl phosphate
Phosphorus can have have expanded octets because it is in the n = 3 row. Typically, phosphorus forms five covalent bonds. In biological molecules, phosphorusis usually found in organophosphates. Organophosphates are made up of a phosphorus atoms bonded to four oxygens, with one of the oxygens also bonded to a carbon. In methyl phosphate, the phosphorus is sp3 hybridized and the O-P-O bond angle varying from 110 to 112o.
Methanethiol & Dimethyl Sulfide
In biological system, sulfur is typically found in molecules called thiols or sulfides. In a thiol, the sulfur atim is conded to one hydrogens and one carbon and is analogous to an alcohol. In a sulfide, the sulfur is bonded to two carbons. In both cases the sulfur is sp3hybridized and the bond angles are much less than the typicall 109.5o.
Methanethiol
Example

Identify geometry and lone pairs on each heteroatom of the molecules given.
Show AnswerSp3 Hybridized Carbon Has

Contributors
Sp3 Hybridized Carbon Examples
- Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
- Prof. Steven Farmer (Sonoma State University)
